Synchron Inc, an endovascular BCI brain-computer interface company, announced the commencement of the US COMMAND EFS, an early feasibility study of its Stentrode motor neuroprosthesis for patients with severe paralysis. The company enrolled the first patient in the COMMAND at Mount Sinai Hospital based in NYC (1).
The company announced that the COMMAND EFS would study the Stentrode MNP, a permanent BCI implant, or the brain chip (2). It conducts the clinical trial under the first IDE, investigational device exemption approved by the FDA to assess the BCI. The FDA had approved clinical studies for BCI in short-term experiments in the past.
We're proud to announce that we've achieved a significant milestone for our company and millions of people living with #paralysis. We've officially enrolled the first patient in our early feasibility study here in the US. To learn more https://t.co/vHRYtUx9z3 pic.twitter.com/XWtwh6kS4n
— Synchron (@synchroninc) May 4, 2022
The NIH, National Institutes of Health, funded EFS primarily aims to assess the implant’s safety and start exploring Synchron’s Stentrode™ quantified efficacy measures in patients with severe paralysis. The aim is to allow the patient to control the digital device hands-free.
Synchron’s recent SWITCH clinical trial demonstrated the technology’s safety in four patients, which the company unveiled last month at the American Academy of Neurology Conference (3).
Professor Bruce Campbell from the University of Melbourne presented the long-term safety data from the trial (4). Researchers had monitored participants for a year and found that the implant was safe and there were zero adverse events leading to any disability or death. In addition, the implant was not migrated, and the implant site remained open.
Following implantation in the SWITCH trial, patients could use the Stentrode system from their homes under no supervision to send texts, do online shopping, and even manage their finance.
“The COMMAND study would progress Synchron’s BCI technology via the feasibility stage as we prepare for our pivotal trial. The first patient we enrolled under an IDE is a major milestone for the entire space as we advance our solution for more than 5 million people in the US living with paralysis,” said CEO and Founder of Synchron, Thomas Oxley (5).
Read Also: Biohacking: An Industry With Opportunities Worth Over $50 Billion
Other BCI Human Trials
Synchron competes directly with Elon Musk’s Neuralink, which is yet to start clinical trials (6).
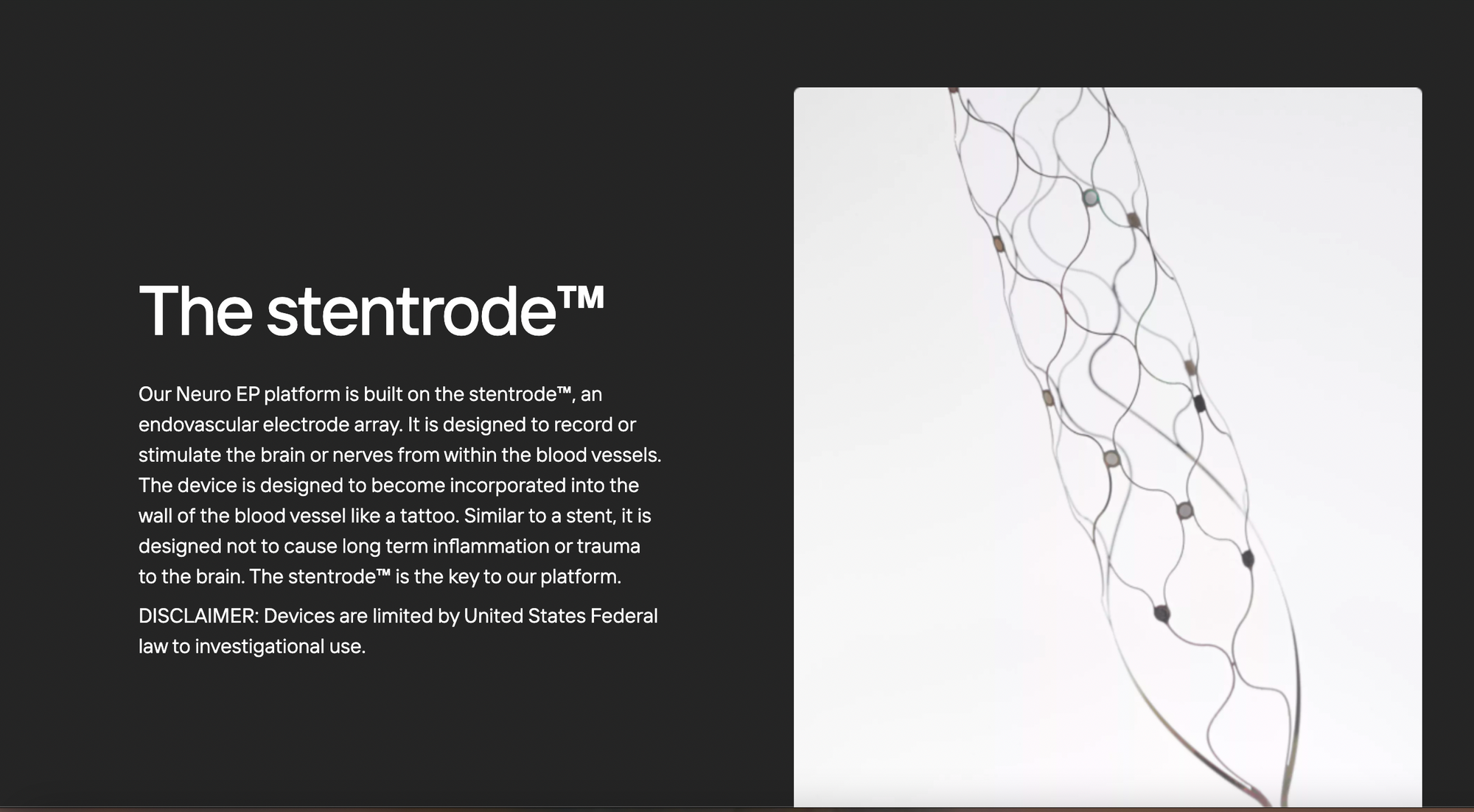
While most brain chips, including Nerualink’s, either sit outside the skill or needs to be directly implanted in the brain, Sunchron’s brain chip travels through blood vessels to reach the brain. Strentrode would then translates brain waves into machine-readable signals that devices like smartphones and computers can detect.
Moreover, the Synchron approach is also different from its peers in pursuing FDA approvals via clinical trials. Companies like Blackrock for its MoveAgain NCI and Neurolutions with its IpsiHand have already received temporary approvals or breakthrough designations (7, 8). It enabled them to reach a few patients without any clinical trials.
However, we believe that though Synchron may take longer to reach the market, its approach would give the company wider access to patients when approved.
Read Also: Lucrative Opportunities in the Fertility Market
The Market
According to Research and Markets report (9), the market for BCI worldwide is likely to grow from 1.9 billion USD in 2021 to 3.3 billion USD by 2026, at an 11.5% CAGR.
The market for BCI in medical applications is likely to grow from 1.4 billion USD to 2.4 billion USD at a 10.9% CAGR during the same period. In comparison, the report predicts that the market of BCI for applications in entertainment and gaming is likely to grow from 99.2 million to 187.2 million USD at a 13.5% CAGR.
The available data also suggests that one in 50 people globally live with paralysis, with more than 5 million people in the US alone. The estimate is that the demand would increase even further if we consider about 500k people suffering from spinal cord injuries every year (10).
If the trial is successful, Stentrode BCI could reach the market within the next decade. However, we are unsure if patients would be comfortable having computer chips in their brains.






